How Does Atomic Radius Change From Left To Right
Learning Objectives
- Define diminutive radius.
- Depict how the atomic changes within a catamenia.
- Describe how the atomic radius changes within a group.
How can all of these people fit in such a small space?
 Events draw large numbers of people to them. Even an outdoor effect can fill upwardly and so that at that place is no room for more people. The crowd chapters depends on the amount of space in the venue, and the amount of infinite depends on the size of the objects filling it. We tin get more than people into a given space than we tin elephants, because the elephants are larger than people. Nosotros tin get more squirrels into that same infinite than we can people for the same reason. Knowing the sizes of objects nosotros are dealing with can be important in deciding how much infinite is needed.
Events draw large numbers of people to them. Even an outdoor effect can fill upwardly and so that at that place is no room for more people. The crowd chapters depends on the amount of space in the venue, and the amount of infinite depends on the size of the objects filling it. We tin get more than people into a given space than we tin elephants, because the elephants are larger than people. Nosotros tin get more squirrels into that same infinite than we can people for the same reason. Knowing the sizes of objects nosotros are dealing with can be important in deciding how much infinite is needed.
The size of atoms is important when trying to explain the behavior of atoms or compounds. I of the means we can limited the size of atoms is with the atomic radius . This data helps u.s. empathise why some molecules fit together and why other molecules have parts that get besides crowded nether sure conditions.
The size of an atom is divers past the edge of its orbital. However, orbital boundaries are fuzzy and in fact are variable under unlike atmospheric condition. In order to standardize the measurement of atomic radii, the distance betwixt the nuclei of two identical atoms bonded together is measured. The atomic radius is defined as half the altitude between the nuclei of identical atoms that are bonded together.

Figure 1. The atomic radius (r) of an atom can exist defined every bit one half the altitude (d) between two nuclei in a diatomic molecule.
Diminutive radii have been measured for elements. The units for atomic radii are picometers, equal to 10−12 meters. As an instance, the internuclear distance between the two hydrogen atoms in an H2 molecule is measured to be 74 pm. Therefore, the atomic radius of a hydrogen atom is [latex]\frac{74}{2}=37\text{ pm}[/latex].
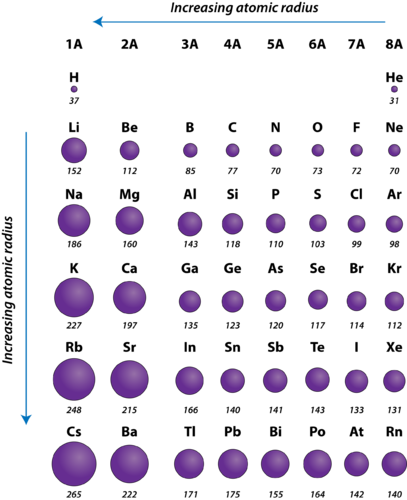
Figure 2. Diminutive radii of the representative elements measured in picometers.
Periodic Tendency
The diminutive radius of atoms more often than not decreases from left to right beyond a flow. There are some pocket-size exceptions, such equally the oxygen radius being slightly greater than the nitrogen radius. Within a menstruum, protons are added to the nucleus as electrons are beingness added to the same chief free energy level. These electrons are gradually pulled closer to the nucleus because of its increased positive charge. Since the force of allure between nuclei and electrons increases, the size of the atoms decreases. The effect lessens as one moves further to the right in a catamenia because of electron-electron repulsions that would otherwise cause the atom'south size to increment.
Group Tendency
The atomic radius of atoms generally increases from acme to bottom within a group. As the atomic number increases down a grouping, there is again an increment in the positive nuclear charge. All the same, in that location is also an increase in the number of occupied principle free energy levels. Higher principal energy levels consist of orbitals which are larger in size than the orbitals from lower free energy levels. The effect of the greater number of principal energy levels outweighs the increase in nuclear charge and then atomic radius increases down a grouping.

Figure 3. A graph of atomic radius plotted versus atomic number. Each successive menses is shown in a dissimilar color. As the diminutive number increases inside a catamenia, the diminutive radius decreases.
Summary
- Atomic radius is determined as the distance between the nuclei of 2 identical atoms bonded together.
- The diminutive radius of atoms generally decreases from left to right across a menses.
- The atomic radius of atoms generally increases from top to bottom within a grouping.
Do
Utilize the link below to respond the following questions:
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Table_of_the_Elements/Atomic_Radi
- What influences the atomic size of an atom?
- What is a covalent radius?
- What is an ionic radius?
Review
- Define "atomic radius."
- What are the units for measurement of atomic radius?
- How does the diminutive radius change beyond a period?
- How does atomic radius modify from top to bottom within a group?
- Explain why the diminutive radius of hydrogen is and then much smaller that the atomic radius for potassium.
Glossary
- atomic radius: The atomic radius is defined every bit half the altitude between the nuclei of identical atoms that are bonded together.
Source: https://courses.lumenlearning.com/cheminter/chapter/periodic-trends-atomic-radius/
Posted by: davishatted.blogspot.com


0 Response to "How Does Atomic Radius Change From Left To Right"
Post a Comment